Terpenoids as presented by Ralph Ikan
Natural Products: A Laboratory Guide, 1969

Introduction
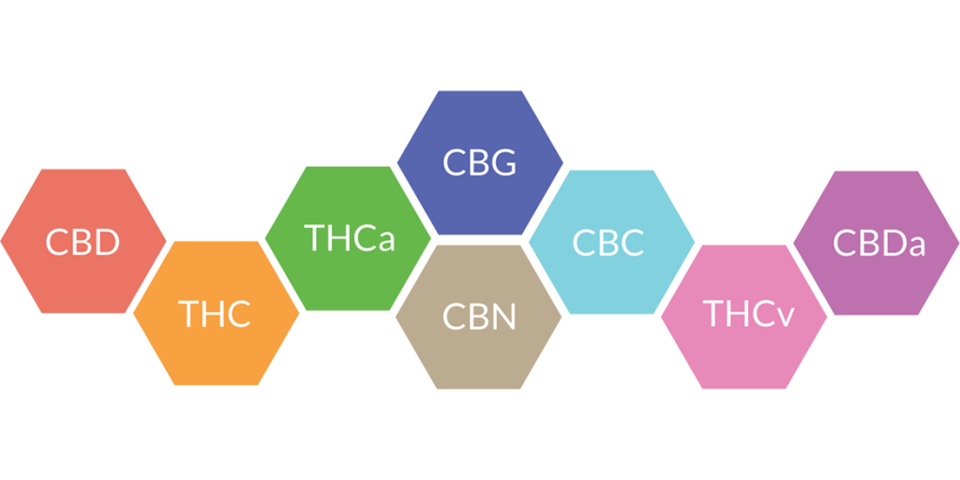
In my previous post, I introduced and applied the concept of cheminformatics to sort and visualize structural similarity of 47 select compounds discussed in a chapter of Marijuana and the Cannabinoids by ElSohly.
I find the chemical details of natural products can be a bit overwhelming to say the least. This is why I open these articles with an overview graphic showing the names and structure similarity relationships of the compounds that will be discussed.
The graphic shown above was created using the bioconductor package fmcsR available for the free and open source R programming language.
Consider it as a road map for your introductory lesson on terpenes and terpenoids.
Today we will take a brief dive into Ralph Ikan's Natural Products: A Laboratory Guide first published in 1969.
We will get our feet wet on the Isoprenoid section terpenoid subsection.
Review
Terpenes and Terpenoids are the topic du jour in the cannabis scene, these are the volatile compounds that give each cannabis strain a unique and memorable taste and smell.
Therefore, we know about terpenes from real life experiences but what are terpenes from the chemical perspective and how are they similar or different from terpenoids?
Ralph Ikan opens this chapter by giving us some rules to define the chemical formula of terpenes and terpenoids.
| Category | Chemical formula |
|---|---|
| Monoterpenes | C10H16 |
| Sesquiterpenes | C15H24 |
| Diterpenes | C20H32 |
| Triterpenes | C30H48 |
| Tetraterpenes | C40H64 |
| Polyterpenes | (C5H8)n |
The chemical formula above indicates that we expect to find terpenes with a certain number of Carbon (C) or Hydrogen (H) atoms.
"Terpenoids are widely distributed in nature, mostly in the plant kingdom. They may be regarded as derivatives of oligomers of isoprene"
Terpenoids are found concentrated in essential oils, which consiste of a complex mixture of terpenes, sesquiterpenes, alcohols, aldehydes, ketones, acids, and esters.
The separation of individual components is accomplished by vacuum fractionation and chromatography.
There are four general methods for isolation of essential oils:
- Expression
- Steam distillation
- Extraction via volatile solvents
- Resorption in purified fats
We will explore several of these categories below. I have included annotations on the odor based on information from The Good Scents Company. In some cases the information is missing as they did not have an entry to my satisfaction.
Acyclic Monoterpenes
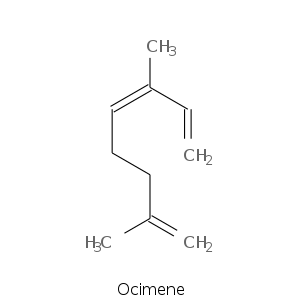
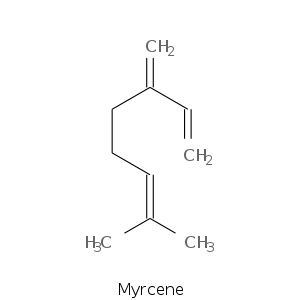
| Terpene | Description |
|---|---|
| Ocimene | green citrus, tropical fruity, woody, pine, metallic, mango nuances, licorice, anise |
| Myrcene | peppery, spicy, balsamic, plastic, woody, fruity tropical mango, leafy |
Aldehydes
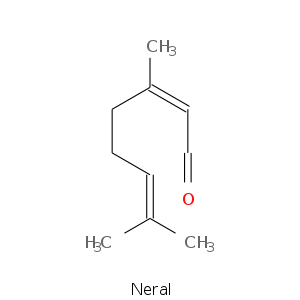
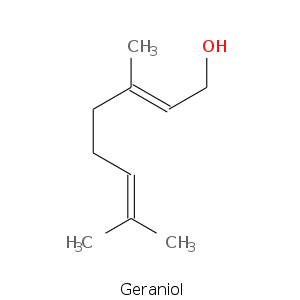
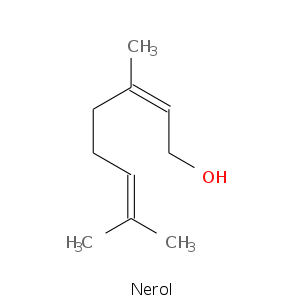
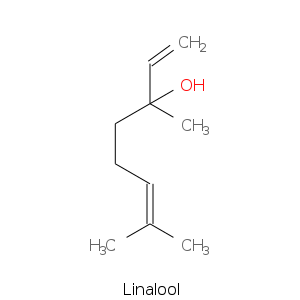
Alcohols
| Terpene | Description |
|---|---|
| Geraniol | sweet, floral, citrus, rose, waxy |
| Nerol | sweet, natural, neroli, citrus, magnolia |
| Linalool | citrus, floral, sweet, bois de rose, woody, green blueberry |
Monocyclic Monoterpenes
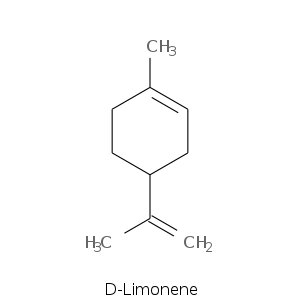
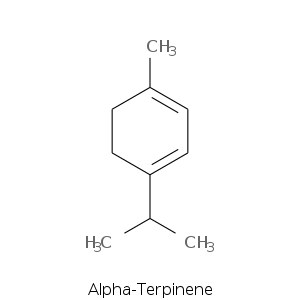
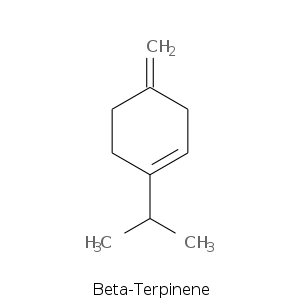
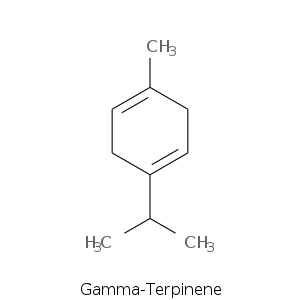
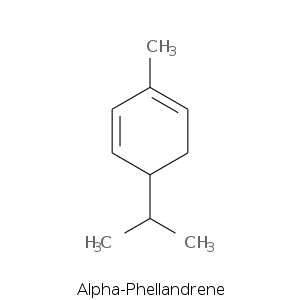
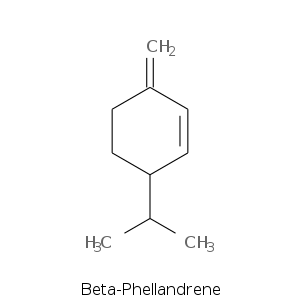
| Terpene | Description |
|---|---|
| Limonene | Citric, fresh, nuances of tangerine, orange peel, celery and lemon oil |
| Alpha-terpinene | Woody, terpene, lemon, herbal, medicinal citrus |
| Beta-terpinene | |
| Gamma-terpinene | Woody, terpinic, lemon, lime, tropical, herbal, sweet citrus |
| Alpha-phellendrene | Citrus, herbal, terpinic, green, woody, pepper, black pepper |
| Beta-phellendrene | Minty, terpinic |
Monoterpene: Alcohols
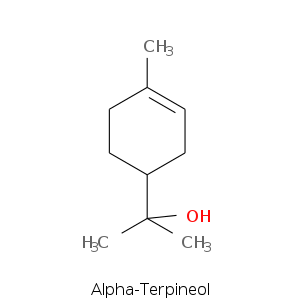
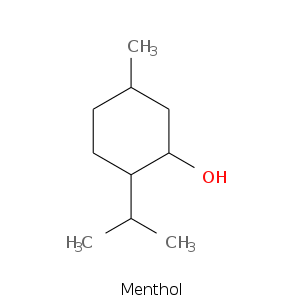
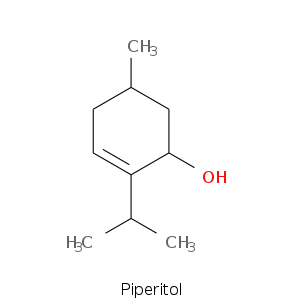
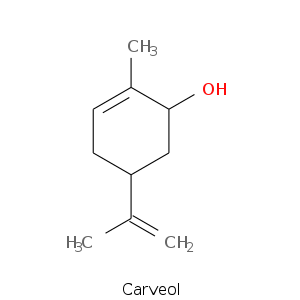
| Terpene | Description |
|---|---|
| Alpha-terpineol | Pine, terpinic, lilac, citrus, woody, floral |
| Menthol | Peppermint, cooling, woody |
Monoterpenes: Aldehydes
Monoterpene: Ketones
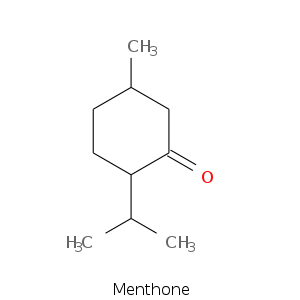
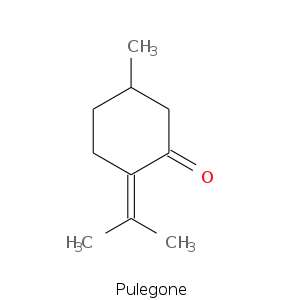
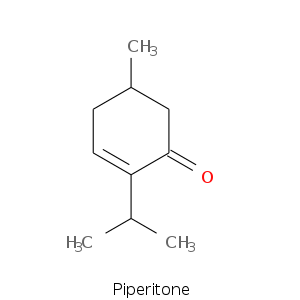
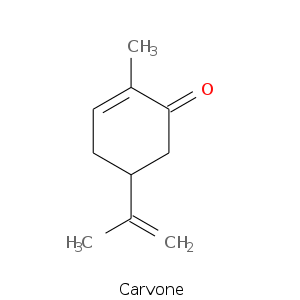
Bicyclic monoterpenes: Thujane Group
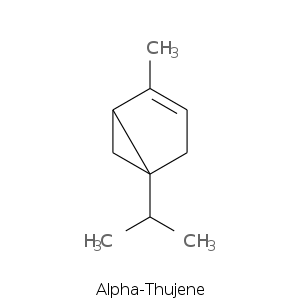
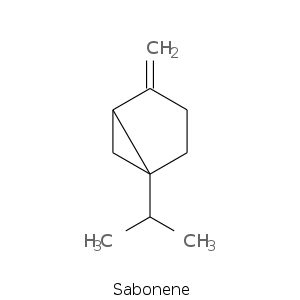
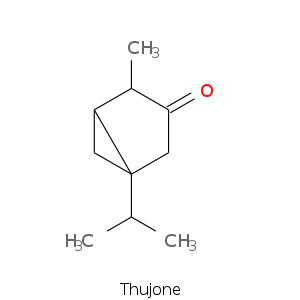
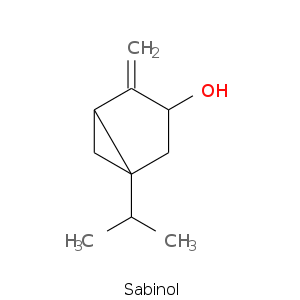
Bicyclic monoterpenes: Carane Group
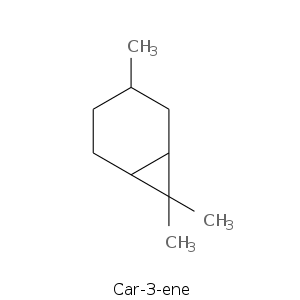
| Terpene | Description |
|---|---|
| Car-3-ene | Citrus, terpinic, herbal, pine, solvent, resinous, phenolic, cypress, medicinal, woody |
Bicyclic monoterpenes: Pinane Group
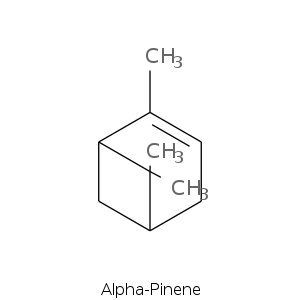
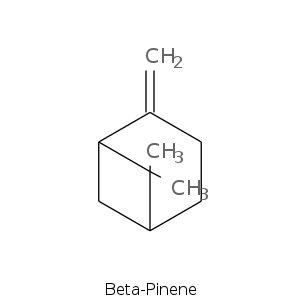
| Terpene | Description |
|---|---|
| Alpha-pinene | Fresh, camphor, sweet, pine, earthy, woody |
Bicyclic monoterpenes: Camphane Group
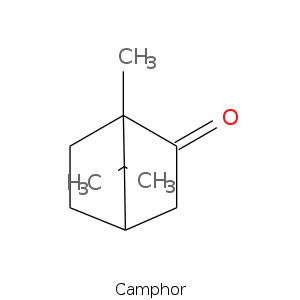
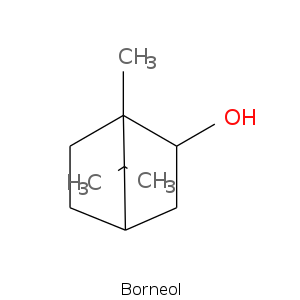
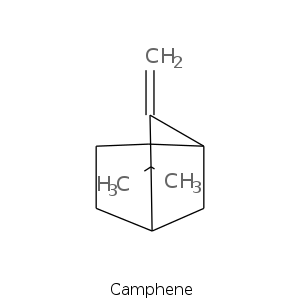
Bicyclic monoterpenes: Fenchane Group
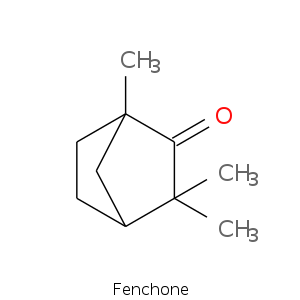
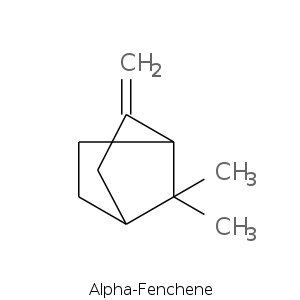
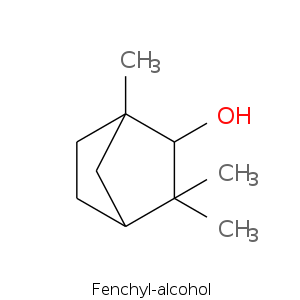
| Terpene | Description |
|---|---|
| Fenchyl alcohol | Camphor, borneol, pine, woody, sweet lemon |
Sequiterpenes
- Derived from the higher boiling fraction of essential oils.
- Formed by the union of three isoprene units.
- Unsaturated compounds that may be acyclic, monocyclic, bicyclic, and tricyclic.
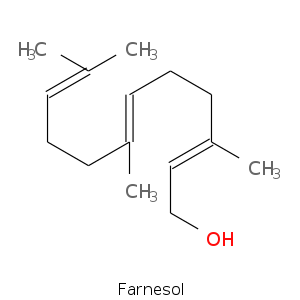
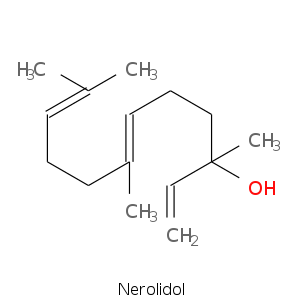
| Terpene | Description |
|---|---|
| Nerolidol | Floral, green, waxy, citrus, woody |
Monocyclic Sesquiterpenes
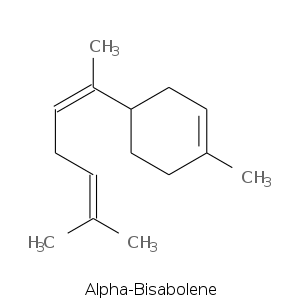
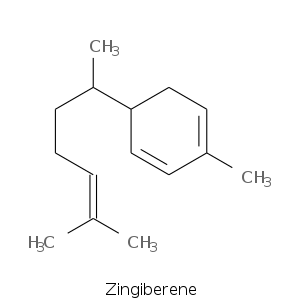
| Terpene | Description |
|---|---|
| Alpha-bisabolene | Balsamic, spicy, floral |
Bicyclic Sesquiterpenes
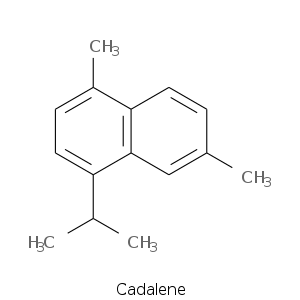
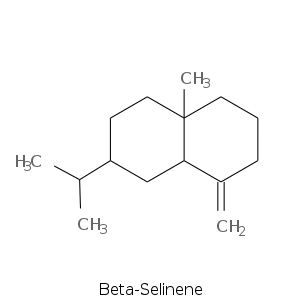
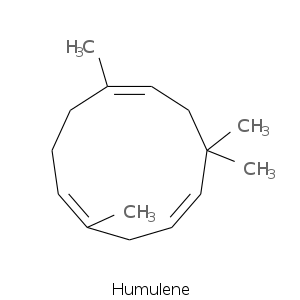
More complex sesquiterpenes
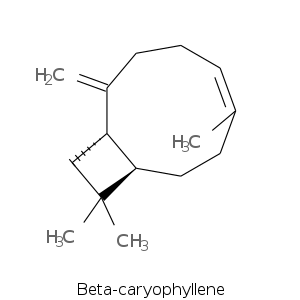
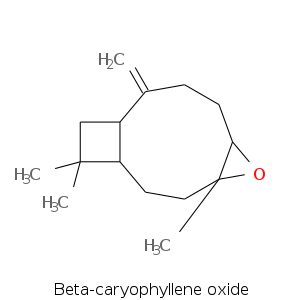
| Terpene | Description |
|---|---|
| Humulene | Woody, oceanic-watery, spicy-clove |
| Beta-caryophyllene | Sweet, woody, spicy, clove, dry |
| Beta-caryophyllene oxide | Sweet, fresh, dry, woody, spicy |
References
Ikan, R. (1969). Natural products: a laboratory guide. Isoprenoid section, terpenoid subsection. Academic Press.